Logistics
- Event: Federal Listening Session on Interoperability of Medical Devices, Data, and Platforms to Enhance Patient Care, A Networking and Information Technology Research and Development (NITRD) Program workshop organized by the Health Information Technology Research and Development (HITRD) Interagency Working Group (IWG).
- Federal Register Notice: 84 FR 29884, Notice of Listening Session on Interoperability of Medical Devices, Data, and Platforms To Enhance Patient Care, National Coordination Office (NCO) for Networking and Information Technology Research and Development (NITRD).
- Meeting Date: July 17, 2019
- Meeting Location: FDA, Great Hall, Building 31, 10903 New Hampshire Ave, Silver Spring, Maryland
- Agenda: HITRD-Listening-Session-Agenda.pdf
- Listening Session Attendees: HITRD-Listening-Session-Attendees.pdf
Rationale
Medical devices, electronic health records (EHRs) and the data generated by and stored in these systems are essential to the practice and advancement of modern medicine and healthcare. If interoperability is enabled between these devices and systems, patient care can be improved using connected and autonomous applications, automated error detection and more rapid development cycles. Achieving safe interoperability will require the system of care delivery to be properly engineered to address both the desired (e.g. new apps and safety interlocks) and undesired (e.g. unsafe interference) emergent properties. Interoperability needs to be engineered for safety.
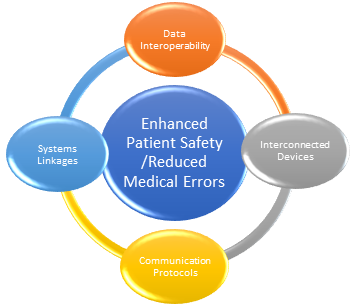 Figure 1:Potential Outcome of Interoperability of Medical Devices, Data, and Platforms
Figure 1:Potential Outcome of Interoperability of Medical Devices, Data, and Platforms
A future scenario could be: People with serious injuries or illness are moved quickly to the best-suited emergency room (ER) with data flowing to the ER in advance of their arrival. Once at the hospital, equipment can be transferred with the patient and seamlessly integrated with the hospital systems with replacements given to the emergency response or exchanged with hospital equipment. These changes are automatically documented with no deficit in patient safety, loss in data fidelity or data security as the patient transitions to a new care location. New devices can be augmented, or devices removed as the patient’s status changes and details of these changes, calibration of the instruments, and equipment’s unique device identifier [UDI] and settings get recorded and synchronized. If a piece of equipment is potentially faulty, it can be switched seamlessly (no loss of function or addition of any hazards) and interoperability with a device from another vendor. Real-time device data and settings from patient medical devices, such as insulin pumps, are identified and logged, and time synchronized and integrated with the medical record. These data will flow dynamically through changes in equipment that occur in moves from emergency to the operating room and later to the intensive care unit, to a rehabilitation facility and finally to home in a seamless manner. This will allow for data and metadata to flow even as changes in equipment are mapped to individual patient needs and environment. Each change in equipment configuration will be documented and stored via the meta-data (e.g., the UDI) from the device. The medical black box recorder-type data can be stored and analyzed to assess the quality of care, review adverse events, create a learning health system for process improvement, create automated/semi-autonomous care algorithms for managing groups of medical devices, or to assess equipment and predict maintenance requirements. This will also improve the consistency and quality of care, create real-time automated care systems, and create a learning health system.
While this vision exemplifies one scenario, there are other healthcare use cases that require fast, data-dependent interactions between multiple systems to enhance care and patient safety. These types of records and the interactions they enable are widely used or are being actively developed in other industries, such as the automotive, aviation and energy sectors, and are being created in medical systems in other countries.
What are the medical device interoperability solutions that are relevant for advancing healthcare and patient safety? Seamlessly flowing interoperable, standardized data from medical devices and systems, when utilized effectively, could significantly enhance patient outcomes, identify and reduce errors, enhance the efficiency of care delivery, reduce development times and costs, improve the quality and consistency of patient care, and decrease healthcare provider burnout.
This listening session builds on previous government efforts by the Food and Drug Administration, the Department of Defense, the Veterans Administration, the National Institute of Standards and Technology, the National Institutes of Health, and the National Science Foundation to advance interoperability by listening to the community explore solutions that promote our shared future vision of next generation, interoperable and intelligent health system powered by technologies commonly used in other industries.
Goals
The listening session will be focused on the following six key areas to advance interoperability:
- Data, meta-data
- Access to control of devices
- Leadership and governance
- Incentives
- Management and modernization of standards
- Infrastructure, Tools, and Use Cases
The listening session will include subject matter experts from industry, academia, and the Federal government. The outcome of the listening session is expected to be a public report that summarizes the event and its key takeaways to advance medical device interoperability.
Documents/Publications/References
- The Interoperability of Medical Devices, Data, and Platforms to Enhance Patient Care, Richard Conroy; Wendy J. Nilsen; Health Information Technology R&D Interagency Working Group, March 2020.
- A Summary of the February 2019 Request for Information: Action on Interoperability of Medical Devices, Data, and Platforms to Enhance Patient Care, HITRD IWG Organizing Committee, July 2019.
- In 2010, the FDA, Continua Health Alliance, and the Center for Integration of Medicine Innovative Technology (CIMIT) sponsored a public workshop to bring together relevant stakeholders and find common ground for interoperability, safety in an interoperable environment, and regulatory pathways.
Presentations
- Interoperability Listening Session: Federal Vision, Sandy Weininger, July 17, 2019.
- Medical Autonomous Systems for Improving Healthcare Delivery, Loretta Schlachta-Fairchild, July 17, 2019.
- The Medical Interoperability Reference Architecture Research Collaboration (video), Defense Health Agency, DOD. Presented by Loretta Schlachta-Fairchild at Federal Listening Session on Interoperability of Medical Devices, Data, and Platforms to Enhance Patient Care on July 17, 2019.

